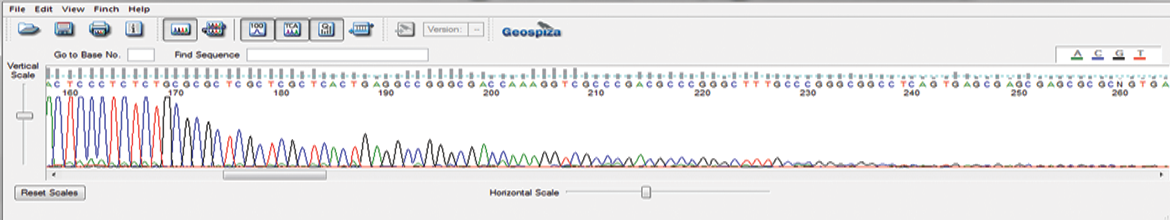
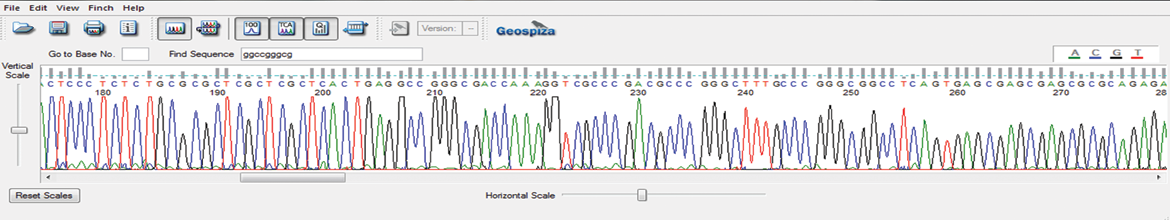
AAV-ITR Sanger Sequencing
GENEWIZ from Azenta’s proprietary Sanger sequencing method sequences through difficult inverted terminal repeat (ITR) regions of adeno-associated virus (AAV), to expedite screening and validation of leads for your cell and gene therapy research.
This newly-developed protocol prevents abrupt reduction in the sequencing signal at the start of the ITR hairpin and reads through the full length of the ITR region. AAV-ITR sequencing can be combined with our Primer Walking service to sequence whole AAV vectors.
GENEWIZ AAV-ITR Sequencing Provides a Breakthrough Solution
Adeno-associated virus (AAV) is one of the most actively investigated gene therapy vehicles. Our robust AAV-ITR Sequencing Protocol sequences through its difficult inverted terminal repeat (ITR) regions to better support your viral vector testing and drug discovery pipeline.
FEATURES & BENEFITS

AAV-ANCED STRATEGIES
For Adeno-Associated Virus in Gene Therapy
✓ Reliable quality control and safety testing for AAV development
✓ Strategies to read through inverted terminal repeats in AAV
✓ High-fidelity methods for AAV plasmid prep and packaging
✓ Advanced sequencing for AAV quality and safety monitoring
Technical Resources

eBook | A Guide to AAV in Gene Therapy
AAV vectors play a critical role in gene therapy, offering an ideal approach for targeted gene delivery. However, understanding the complexities of AAV packaging, optimization, and quality control testing can be challenging. In this eBook learn step-by-step strategies for mastering AAV vector development and enhancing your gene therapy applications.

Tech Note: An Efficient and Reliable Approach to AAV Packaging
While AAV is a powerful vehicle for gene delivery, unstable ITRs threaten the fidelity of rAAV genomes. Loss of ITR integrity diminishes the packaging efficiency of rAAV particles and thus viral titer. In this tech note, discover the effect of partial and full ITR deletions on AAV packaging and how to reliably identify and effectively correct them.

Tech Note: Reading Through the Inverted Terminal Repeats (ITRs) of Adeno-associated Virus (AAV)
GENEWIZ has developed a high-quality, direct Sanger sequencing method that reads through both intact and commonly mutated inverted terminal repeat (ITR) regions of adeno-associated virus (AAV). This method is an effective tool for assessing the integrity of ITRs in AAV plasmids.

Article: Optimizing AAV Plasmid Preparation and ITR Sequencing
The adeno-associated virus (AAV) is a powerful vehicle for gene therapy; however, working with AAV vectors can be challenging. Spontaneous mutations in the inverted terminal repeat (ITR) regions can accumulate during plasmid propagation in bacteria. Find out how our scientists have optimized the AAV plasmid preparation process to deliver 100% accurate constructs.